List of doctors and approaches: Difference between revisions
| Line 418: | Line 418: | ||
Experimental drugs tend to be very difficult to get, so they may not be very relevant to patients. They generally do not have an established safety track record. | Experimental drugs tend to be very difficult to get, so they may not be very relevant to patients. They generally do not have an established safety track record. | ||
=== Lists of clinical trials === | |||
https://crunchme.notion.site/4d00e39f9f874a4c85cb9046149aeba7?v=2c3284eb773a403e817db9598480a768 | |||
=== Baractinib === | === Baractinib === | ||
Latest revision as of 03:43, 27 April 2024
Approaches tested in randomized controlled trials[edit]
Note: these studies are not necessarily reliable.
HBOT (Hyperbaric oxygen therapy)[edit]
Shamir Medical Center in Israel funded a randomized controlled trial of HBOT on Long COVID patients. It compared HBOT at 2ATA (with oxygen) to 'sham' HBOT that was as mild as possible to give the impression of HBOT treatment. Both the high-pressure HBOT group and the 'sham' control group experienced a few cases of barotrauma. The blinding was fairly good as "the correct group allocation perception rate was 54.1% and 66.7% (p=0.271) in the HBOT and control groups respectively".
One strength of the trial was that endpoints were pre-registered (NCT04647656) to ensure that the results are reliable and free of data mining. The trial met its primary endpoint of cognitive improvement as measured by Neurotrax, a computerized set of tests that objectively measures cognitive performance.
How to get more information: The study results paper can be found at DOI:10.1038/s41598-022-15565-0. The researchers have published additional analyses on functional and structural connectivity and myocardial function.
Ongoing studies: Anders Kjellberg in Oslo Norway is conducting a RCT on Long COVID patients (NCT04842448).
Antihistamines[edit]
An Iranian team lead by Momtazmanesh (DOI:10.1016/j.jpsychores.2023.111389) studied patients who were recovering from COVID patients at a care center after discharge. Of the 98 post-hospitalization patients who were assessed for eligibility, 58 were randomized into the trial. This cohort may not necessarily reflect the type of Long COVID where patients experience a debilitating syndrome characterized by a multitude of symptoms. Some COVID infections were diagnosed by CT scan and symptoms rather than PCR (Iran had a shortage of PCR tests). This randomized controlled trial found greater improvements in those treated with famotidine across the four endpoints (Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Hamilton Depression Rating Scale (HAM-D), and Hamilton Anxiety Rating Scale (HAM-A)). The two groups did not differ in the frequency of adverse effects, suggesting that famotidine had good safety in this cohort. One unusual aspect of the Iranian team is that Shahin Akhondzadeh is one of the major players in the team (and the person who secured funding for the study). Shahin Akhondzadeh has been involved in multiple RCTs that have found a benefit for the intervention tried. Akhondzadeh's consistency in research success is unusual as the primary endpoints in his studies typically reach statistical significance.
Glynne and colleagues (DOI:10.1136/jim-2021-002051) studied a cohort of Long COVID patients (along with a convenience sample of vaccinated healthy controls). Their prospective observational study found some improvement from histamine receptor antagonists (antihistamines).
Phyto-V phytochemical capsule (undisclosed conflict of interest)[edit]
Professor Robert Thomas is the lead author of various papers on Phyto-V such as this placebo-controlled trial paper (published in March 2022). The paper states that "The authors declare no conflict of interest". Nonetheless, the paper thanks Keep-Healthy.com for the donated probiotics; the Keep-Healthy.com website lists Robert Thomas as a "medical advisor" (archive.is). The same website has also advertised consultation services offered by Thomas (archive.org).
Health Education Publications is listed as the owner of Keep-Healthy.com and is also the publisher of a 2010 book by Robert Thomas (amazon.com, archive.ph).
An earlier Nov 2021 paper by Robert Thomas (and other co-authors) discusses a non-controlled study on YourGutPlus+. The products were "donated free by Keep-healthy Ltd, Isle of Man". The disclosures section in that paper states:
This was a non-commercial trial and no direct funding has been received from external organisations although the probiotics was supplied free of charge to the trials unit as mentioned above. The research team involved in the study were not being paid to recruit patients into the study, had no other financial incentives and have no connection with the manufactures.
SIM-01 synbiotic / probiotic[edit]
- https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(23)00685-0/fulltext
- Pre-registration: https://clinicaltrials.gov/study/NCT04950803
Participants were assigned between June 25 2021 and August 12 2022. On Feb 7 2023, the pre-registration record was changed to reflect the new primary endpoint, from "A composite outcome of any comorbidities [...]" to "alleviation of symptoms or complication of post-COVID conditions within 6 months". The Lancet paper does not mention the word 'sepsis', which was part of the original primary endpoint/outcome. This suggests that the goalposts may have been shifted after the trial had begun.
The patients may not have the same "Long COVID" that affects those in support groups with a long list of symptoms and high severity. Patients were diagnosed with PACS (Post-acute COVID-19 syndrome) according to the US criteria. Inclusion criterion was the presence of at least one of 14 PACS symptoms for 4 weeks or more after confirmed SARS-CoV-2 infection, including fatigue, memory loss, difficulty in concentration, insomnia, mood disturbance, hair loss, shortness of breath, coughing, inability to exercise, chest pain, muscle pain, joint pain, gastrointestinal upset, or general unwellness. A previous paper by the lead author (Raphaela Iris Lau) stated that "the prevalence of PACS was 78.7% at an average of 14-month (IQR 11–18 months) follow-up" in a study unrelated to SIM-01.
IVIG for POTS[edit]
This first randomized controlled trial of IVIG in POTS found no difference in symptom response compared to albumin infusion. Both groups showed improvement possibly related to volume expansion obscuring other benefits. Future clinical trials may benefit from the use of POTS-specific clinical outcome measures sensitive to symptoms other than orthostatic intolerance.
https://www.researchsquare.com/article/rs-3500596/v1
Exercise for Post COVID Condition[edit]
In this study, nonhospitalized patients with PCC generally tolerated exercise with preserved cardiovascular function but showed lower aerobic capacity and less muscle strength than the control group. They also showed signs of postural orthostatic tachycardia and myopathy. The findings suggest cautious exercise adoption could be recommended to prevent further skeletal muscle deconditioning and health impairment in patients with PCC.
Patients with PCC reported more muscle pain after HIIT and concentration problems after MICT and had lower aerobic capacity and less muscle strength; 62% showed myopathic signs.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2817149
Note: these results may be dubious as the majority of patients report negative experiences with exercise.
Doctors treating long haul COVID and vaccine injury (post vaccination syndrome)[edit]
This is a list of doctors and doctor groups treating long haul. These health problems are new so there hasn’t been much research about what’s safe and effective. Please become informed about the risks of medical experimentation!
LDN (Low dose naltrexone)[edit]
An Irish research group tested LDN on patients from the Long COVID clinic at Mater Misericordiae University Hospital. While the study suggests a benefit to LDN, the study had no control group.
- Safety and efficacy of low dose naltrexone in a long covid cohort; an interventional pre-post study - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9250701/
FLCCC[edit]
Key treatments: Ivermectin, a statin (atorvastatin), prednisone, fluvoxamine, and many other drugs. For MCAS (mast cell activation syndrome, which a number of long haulers have): low-dose naltrexone (LDN) and various drugs
Theory: Ivermectin's mechanism of action is unclear. It seems to work for long haul but not in all sufferers. Ivermectin may be beneficial because it binds to the spike protein, perhaps preventing an autoimmune reaction that occurs in some people but not others. It may also affect the way the immune system functions (immunomodulatory), which could explain why ivermectin applied on the skin (FDA approved as Soolantra) works for rosacea.
Dr. Been’s youtube channel has talks about fluvoxamine, MCAS (e.g. chats with Tina Peers and Lawrence Afrin), long haul, etc. etc.
His talk with Bruce Patterson and Ram Yogendra may explain why maraviroc is listed as a third-line treatment in the Jan 20222 protocol: https://odysee.com/@DrMobeenSyed:1/long-covid-discussion-with-dr.-bruce:f?r=9U5LyeCuf2jpinYRxYjvNdaM6nQqsThF As there has been a public falling out between Eric Osgoode and the FLCCC (partially over ivermectin), the FLCCC may shift away from treatments advocated by the Bruce Patterson/IncellDX group and their affiliated concierge practitioners.
His talk with Keith Berkowitz on the FLCCC channel discusses LDN and the Jan 2022 I-RECOVER protocol: https://odysee.com/@FrontlineCovid19CriticalCareAlliance:c/FLCCC-WEBINAR-020222_FINAL-EDIT:e?r=9U5LyeCuf2jpinYRxYjvNdaM6nQqsThF
How to get more information: The I-RECOVER protocol can be found at https://covid19criticalcare.com/covid-19-protocols/i-recover-protocol/
A list of doctors friendly to the FLCCC protocols can be found here: https://covid19criticalcare.com/network-support/the-flccc-alliance/
Does it work? What we know so far: Dr. Been talks to many other doctors and aggregates their shared knowledge and experience.
Aciclovir / Acyclovir[edit]
We report a series of four cases depicting the successful use of acyclovir in the treatment of the virus SARS-CoV-2 in patients with long-haul symptoms, especially those in the realm of encephalopathy and neurological problems. Treatment with acyclovir in these patients resolved their symptoms and lowered their IgG and IgM titers, supporting the use of acyclovir as a safe and effective treatment for COVID-19 neurologic symptoms. We suggest the use of the antiviral medication, acyclovir, as a treatment for patients with long-term symptoms and unusual presentations of the virus, such as encephalopathy or coagulopathy.
Guanfacine and NAC[edit]
Yale researchers suggest that the combination can relieve brain fog in Long COVID patients.
https://medicine.yale.edu/news-article/potential-new-treatment-for-brain-fog-in-long-covid-patients/
Bruce Patterson / CovidLongHaulers.com group[edit]
Key treatments: A statin (pravastatin), maraviroc or other CCR5 antagonist, ?ivermectin?, avoiding exercise that makes you sweat, baby aspirin
Testing: An inflammatory marker panel invented by Patterson’s company IncellDX. The panel was designed to specifically measure acute COVID and long haul COVID (PASC).
Their theory: For some reason, the body cannot clear the S1 portion of spike protein from the body. It is the root cause of long haul through hyperimmunity, autoimmunity, or some combination of both hyperimmunity and autoimmunity. Hyperimmunity is when the immune system is overly active, leading to inflammation in blood vessels that cause the body to dysfunction. Autoimmunity is when the immune system starts attacking its host.
Since Patterson’s company makes medical tests, their approach is geared around “precision medicine”. The idea is to test the patient to figure out what’s wrong and to use those tests to guide treatment. That’s the theory anyways. In practice, their concierge service doctors may not necessarily practice precision medicine, e.g. they may recommend maraviroc even if CCL5 is not elevated.
For more information: Search Youtube for drbeen bruce patterson. You’ll find talks between Dr. Mobeen Syed and Dr. Bruce Patterson about the treatment of long haul. You can sign up for testing at covidlonghaulers.com
Statin and maraviroc effectiveness[edit]
On a podcast with Dr. Drew, Patterson and Eric Osgood briefly discuss the trial design of their anticipated clinical trial. The design of the trial anticipates/suggests that many patients won’t respond to a statin plus maraviroc. Osgood draws from the lessons learned from clinical trials studying chronic pain:
...chronic pain is a very difficult uh condition to study because a lot of the study drugs are only going to work on maybe 30 or so percentage of the- of that particular disease population and then it is so prone to the placebo effect as well as to people maybe not being that precise or accurate in the way that they report and so there is a need often to do enriched designs or enriched enrollment designs in order to better demonstrate proof of concept...
A paper put out by the Patterson group (Targeting the Monocytic-Endothelial-Platelet Axis with Maraviroc and Pravastatin as a Therapeutic Option to Treat Long COVID/ Post-Acute Sequelae of COVID (PASC) https://www.researchsquare.com/article/rs-1344323/v1) found a statistically significant benefit to pravastatin plus maraviroc (n=18). However, the larger Cytodyn RCT on the CCR5 antagonist leronlimab (n=56) did not find a statistically significant benefit. See the leronlimab section for more information on leronlimab.
The Patterson group study had a retrospective observational design and would be less reliable than a RCT (randomized controlled trial). The lengthy survey process used in the Patterson group study may heavily bias the results because maraviroc+statin non-responders may not necessarily spend time on the phone completing lengthy questionnaires, especially if they have cognitive difficulties from long haul. The paper does not describe the drop-out rate. It also does not fully describe how 18 patients were selected out of the thousands of patients that have done an IncellDX panel test.
Mast Cell Activation Syndrome specialists[edit]
Theory: Overly active mast cells are responsible for a wide range of symptoms.
MCAS is a syndrome that has been researched for many years before COVID. While it is difficult to diagnose and treat, experimentation with various drugs (some of them are sold over-the-counter) may yield something that will alleviate the patient's symptoms. MCAS seems to be common in long haulers.
How to get more information: A MCAS diagnosis and treatment guideline by Afrin et al. can be found at https://doi.org/10.3109/07853890.2016.1161231
Youtube has many interviews with MCAS specialists such as Lawrence Afrin, Theoharis Theoharides and Tina Peers.
The patient support organization The Mast Cell Disease Society has a list of medical centers treating MCAS at https://tmsforacure.org/resources/finding-a-physician/
Ivabradine for POTS[edit]
A study on 55 patients looked at POTS following COVID-19. The poster presentation can be found at https://www.jacc.org/doi/full/10.1016/S0735-1097%2823%2900500-4
Results: 78% of the patients reported significant improvement of the symptoms within 7 days of ivabradine therapy. Comparing 24-hour heart rate, and HRV time and frequency domains before and after ivabradine therapy, 24-hour heart rate (minimum, average, and maximum) was significantly lower (p-value<0.0001*, =0.001*, <0.0001* consecutively).
Ivabradine will likely be studied as part of RECOVER-AUTONOMIC as part of the NIH's RECOVERY initiative (see the press release).
HELP Apheresis[edit]
Key treatment: Apheresis. Available in Germany and Cyprus.
Theory: Apheresis removes microscopic blood clots that are causing health problems.
Published case studies, patient anecdote: A paper by Beate Jaeger and colleagues claimed excellent results from HELP apheresis:
Of these 17 treated patients, 16 patients felt immediate improvement and 12 patients nearly reached full recovery after completion of the treatment. A 6–10-month follow-up revealed that 15 patients maintained their improvements. Thus, of the 17 patients with severe Long COVID symptoms, 16 patients had experienced a great benefit.
One of the co-authors of the paper is Asad Khan, a Long COVID sufferer who underwent HELP apheresis himself. In a June 13 2022 tweet, he described himself as "unwell" and unable to return to his former job as a doctor:
I cannot recommend or discourage any particular treatment. I am better than before but still unwell. I haven’t worked since Nov ‘20. Dr Jaeger saved my life but I do not have any official status in her clinic. I am a normal fee-paying patient there. 2/n
It is possible that HELP apheresis ultimately resulted in the death of a patient. A ME/CFS patient went to Cyprus, significantly deteriorated following treatment, and committed suicide some months later.
- Facebook post where Jack talks about his future plans to go to Cyprus
- Tweets about his passing.
- Facebook posts about his passing.
How to get more information: You can search Facebook for groups with “apheresis” in their name.
Gez Medinger’s Youtube channel has a talk with his friend Dr. Asad Khan about this treatment. https://youtu.be/rEJDjfj7oi8
A youtube interview with Resia Pretorius has a brief segment on HELP apheresis. https://youtu.be/C8tzTmVwEpM
Microclots / Etheresia (Resia) Pretorius and colleagues[edit]
Key treatment: A combination of drugs is used to reduce microclots in long COVID patients: Clopidogrel, Aspirin, a direct oral anticoagulant (Apixiban), plus a proton pump inhibitor (pantoprazole). “Such a regime must only be followed under strict and qualified medical guidance to obviate any dangers”; Resia warns that the therapy carries (significant) risk and should only be done under proper medical supervision. Patients were pre-screened with medical tests (e.g. Thromboelastography) to determine if microclots existed and to determine if the patient was a candidate for therapy.
Theory: Microclots (tiny blood clots) are found in long COVID patients and are the source of major symptoms that they experience. Academic papers on this subject include:
- Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin https://doi.org/10.1186/s12933-021-01359-7
- Huynh, A., Kelton, J.G., Arnold, D.M. et al. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 596, 565–569 (2021). https://doi.org/10.1038/s41586-021-03744-4
For more information: Douglas Kell maintains a website with their group's latest thinking on microclots: http://dbkgroup.org/longcovid/
There is a Youtube interview with Dr. Resia Pretorius on LongCovid microclots (spike protein, HELP apheresis + other topics) https://youtu.be/C8tzTmVwEpM
Pretorius and her colleagues have published a pre-print on the therapy they tried on 24 patients: https://www.researchsquare.com/article/rs-1205453/v1 The clinical data was retracted in the second version of the pre-print. Nonetheless, the second version of the pre-print calls for randomized controlled trials without mentioning the clinical data that the researchers had previously collected.
Does it work? What we know so far: Version 1 of the pre-print strangely contains very little data on how much patients improved on the therapy. The abstract claims: “Each of the 24 treated cases reported that their main symptoms were resolved and fatigue as the main symptom was relieved, and this was also reflected in a decrease of both the fibrin amyloid microclots and platelet pathology scores.”
Survey data from r/CovidLongHaulers shows that 11/22 (50%) of poll respondents reported small or significant improvement in symptoms from blood thinners, substantially below the 100% reported in the pre-print.
A newer March 2023 pre-print publishes data on 91 South African Long COVID patients. While the abstract does not provide any numbers related to the patients' claimed improvement, it concludes: "triple anticoagulant therapy represents a promising treatment option that appears to be highly efficacious, and warrants controlled clinical studies".
Berlin Cures / Gerd Wallukat[edit]
Key treatment: BC 007 (experimental drug), removal of antibodies from the blood via apheresis
Testing: Auto-antibody test. They are one of three German labs providing novel auto-antibody tests (see here).
Theory: Autoimmunity. The body is producing autoantibodies that engage in “friendly fire” and attack specific host tissues instead of foreign antigens (invaders and other things that shouldn’t be in the body).
Resources regarding this group’s work:
https://www.fau.eu/2021/07/06/news/medication-for-autoantibodies-also-effective-for-long-covid/
https://youtu.be/XHaDCBXWArU - “...we assume that the immunoadsorption or plasmapheresis [which get rid of antibodies that cause health problems] represent a new therapeutic option to treat patients…”
Takashi Yamamura[edit]
Key treatments: corticosteroids (e.g. prednisone), IVIG, and treatments used for rheumatoid arthritis (there are a lot of them).
Theory: It is likely that there is some sort of autoimmune component associated with long haulers, e.g. researchers can measure auto-antibodies in patients. In theory, doctors could take treatments that work on classic autoimmune conditions like rheumatoid arthritis and apply those treatments to long haulers. (In practice, Bruce Patterson reports that low-dose corticosteroids seem to work better than high-dose. That isn’t the case with classic autoimmune conditions.)
Classic autoimmune conditions are regularly treated by suppressing the immune system with drugs. IVIG is also thought to be beneficial for autoimmune conditions.
Avindra Nath (NIH) and Takashi Yamamura discuss ME/CFS and long COVID in a discussion available on Youtube: https://youtu.be/xMFCNtoZWIw?t=1096
IVIG effectiveness[edit]
A report by the Canadian government-funded organization CADTH summarizes various meta-analyses on the off-label use of IVIG. Most of the systematic reviews found that IVIG did no better than placebo. Two randomized controlled trials of IVIG on postpolio syndrome found no statistically significant benefit to IVIG compared to placebo, with the possibility of some treatment-related harm in the IVIG group.
IVIG was also no better than placebo for postpolio syndrome and reporting of adverse events was lacking
A series of literature reviews demonstrate mixed and unclear results when it comes to the use of IVIG for treating ME/CFS, a disease arguably similar to Long COVID and Post COVID Vaccination Syndrome. The review titled Back to the Future? Immunoglobulin Therapy for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome by Helen Brownlie and Nigel Speight summarizes four IVIG studies undertaken with ME/CFS patients. The authors note that the RCT data was mixed and inconclusive. However, they argue that it is possible to identify a subset of patients (based on markers of immune dysfunction) where IVIG would be highly effective.
Many patients who respond reach remission up to a year after infusion: “The results of this study demonstrate that a significant proportion of patients (43%) with well-characterized, severe, and long standing CFS responded to high-dose intravenous immunoglobulin therapy”. All responders had improved considerably: “This response was characterized by recommencement of employment, leisure, and social activities, as well as by a significant reduction in physical and psychologic morbidity and by an improvement in cell-mediated immunity.”
Various articles in scientific journals advocate for the use of IVIG, as listed on the IVIG approval resources page.
The NIH believes that there may be some merit to IVIG and corticosteroids in the treatment of long COVID patients. A Science magazine article notes:
Some of the patients who spoke with Science say medications that tamp down the immune system have offered at least a measure of relief. Nath noticed the same phenomenon. He hopes results from an NIH clinical trial testing IVIG and intravenous steroids in Long Covid patients “will be applicable to the vaccine-related complications.” None of the patients with whom Science spoke has fully recovered.
The NIH has funded a phase 2 proof of concept clinical trial on the use of IVIG in Long COVID patients (NCT05350774). Results were expected in 2023 but the timeline has been pushed to 2024.
Thompson et al. (DOI:10.3389/fimmu.2022.1033651) claim that "Long-term high-dose immunoglobulin successfully treats Long COVID patients with pulmonary, neurologic, and cardiologic symptoms".
Rituximab effectiveness[edit]
It is unclear if rituximab is being used in long haulers, although it is a drug used to treat rheumatoid arthritis. For ME/CFS, rituximab did not show a benefit in the 151 patients randomized in a controlled trial (see https://doi.org/10.7326/M18-1451).
NIH, Farinaz Safavi, Avindra Nath - IVIG, corticosteroids, PLEX[edit]
Key treatments: IVIG, corticosteroids, PLEX
Theory: Safavi et al. believe that immune dysregulation may occur following vaccination (and does occur following COVID-19 infections).
Results: A 2022 note published by Oaklander, Nath, and colleagues discusses treatment of Long COVID with IVIG and corticosteroids; "Longitudinal improvement averaged 52%, although none reported complete resolution."
A pre-print on post-vax patients by Safavi et al. claims that the patients that they treated had complete or near-complete improvement after two weeks. Patients seen at the NIH such as Danice Hertz have publicly disputed the claims of recovery.
Nath had previously discussed COVID vaccine injury in a May 16 2021 interview with Body Politc at the 25:46 mark. At the time, he did not characterize COVID vaccine injury as something that is easily treatable. He did not describe patients becoming fully or almost completely recovered. A conversation between Nath and Takashi Yamamura (Nov 2021) also did not mention miraculous recoveries in people suffering from COVID vaccine injuries.
Nath has subsequently published an article in the American Academy of Neurology's journal promoting immunotherapies like PLEX (plasmapheresis).
Corticosteroids[edit]
Charite University in Germany will study the 10-40% of mild COVID-19 patients who experience persisting or new symptoms known as post-COVID-19 syndrome (PCS). (Those patients may be quite different than those in Long COVID support groups.) Their study will examine the effect of corticosteroids (4 weeks + 2 week taper) on patients with cognitive deficits.
https://classic.clinicaltrials.gov/ct2/show/NCT05986422
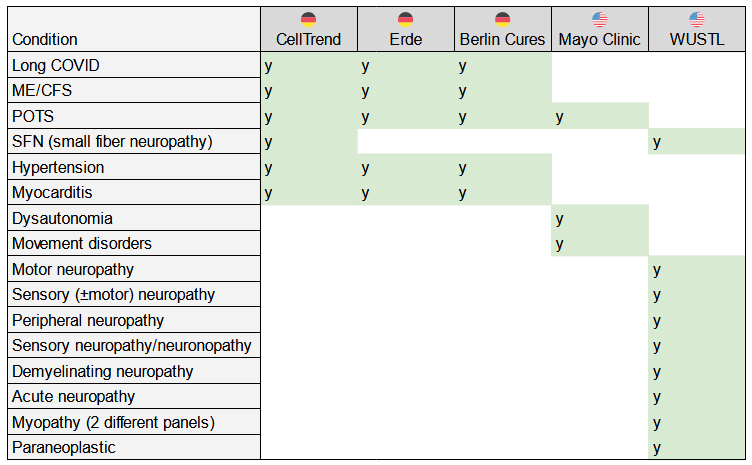
Non-standard auto-antibody tests[edit]
The following chart shows disorders and the labs offering tests to determine if there are auto-antibodies related to those conditions. Auto-antibodies would suggest that the condition is being caused by the body attacking itself. If so, existing treatments for autoimmunity may be helpful.
See the page on non-standard auto-antibody tests for more information.
Plasma exchange / apheresis[edit]
Theory: Whereas intravenous immunoglobulin (IVIG) therapy injects donated antibodies into a patient, plasma exchange will remove the patient's existing antibodies first before replacing them with donated antibodies. The theory is that problematic auto-antibodies are removed and replaced with non-pathogenic antibodies from donors. Plasma exchange is usually combined with a therapy to suppress the immune system so that the patient does not create more problematic auto-antibodies.
Note that IVIG contains auto-antibodies too because healthy donors have auto-antibodies, but at lower rates than unhealthy people (see https://www.jaad.org/article/S0190-9622(08)00939-0/fulltext#relatedArticles).
Plasma exchange is known by other names such as apheresis, plasmaphersis, TPE (therapeutic plasma exchange), PE, PET (PE therapy), and PLEX. It is not the same as HELP apheresis. HELP apheresis substitutes clotting-related components of the blood (and other things such as pathogens associated with those portions of the blood) while plasma exchange focuses on substituting the antibody-related components of the blood.
More information:
- A Youtube video from Yale Medicine explains apheresis: https://www.youtube.com/watch?v=8mnOUoI3bDk&ab_channel=YaleMedicine
- Avindra Nath, a researcher at NIH, talks about long COVID treatment: https://youtu.be/YpfHoz22ePk?t=1952
- A letter to the editor discusses PE as a treatment for encephalitis (brain inflammation) caused by acute COVID-19: https://doi.org/10.1093/brain/awaa337
Gustavo Aguirre Chang[edit]
Key treatments: ivermectin, monoclonal antibodies, HELP apheresis
Theory: Monoclonal antibodies treat a persistent SARS-CoV-2 infection that is causing long COVID. Ivermectin is an antiviral that works against SARS-CoV-2.
More information:
monoclonal antibody paper: https://www.researchgate.net/publication/355806161_INCLUSION_OF_MONOCLONAL_ANTIBODIES_AGAINST_THE_VIRAL_LOAD_IN_THE_FIRST_LINE_OF_THERAPEUTIC_ACTION_FOR_CHRONIC_COVID_LONG_COVID
Ivermectin paper: https://www.researchgate.net/publication/344318845_POST-ACUTE_OR_PROLONGED_COVID-19_IVERMECTIN_TREATMENT_FOR_PATIENTS_WITH_PERSISTENT_SYMPTOMS_OR_POST-ACUTE
Interview with Long Covid Apheresis Community (Youtube)
David Putrino / Physical therapy[edit]
Key treatment: Physical therapy for dysautonomia
Theory: Physical therapy can help ‘retrain’ the body’s autonomic nervous system (ANS). The ANS controls certain bodily functions without you having to think about it. For example, if you start doing exercise, your heart rate will go up and you will breathe more.
- Note: The ME/CFS community would argue that graded exercise therapy (GET) has caused more harm than good in people with ME/CFS. Physical therapy for long COVID could have similar problems. Putrino is aware that exercise causes symptoms to flare in long COVID patients so his treatment involves low levels of exercise that the patient can handle.
Putrino has multiple interviews on Youtube such as this one: https://youtu.be/ggyOnEpM0x8
Bisaccia, Ricci, Fedorowski, Gallina, and colleagues / dysautonomia treatments[edit]
This group published a review paper on PASC / long COVID. It discusses dysautonomia treatment: https://www.mdpi.com/2308-3425/8/11/156/pdf
Key treatments (for dysautonomia): Non-pharmacological measures should be considered as first-line treatment options
- physical reconditioning with aerobic progressive exercise training programs
- compression garments / stockings
- liberal intake of water and salt
- drinking water before getting up in the morning
- sleeping with the head of the bed elevated
- careful avoidance of situations that can exacerbate symptoms (sleep deprivation, heat exposure, alcohol intake, or large or heavy meals)
- Physical maneuvers such as leg crossing, muscle tensing, and squatting have been shown to be effective in delaying/preventing vasovagal syncope if used at the onset of prodromal symptoms.
Pharmacological therapies have been frequently used in PASC patients (see Table 3 in their paper). These should be reserved for patients which do not respond to nonpharmacological therapies and are complementary to nonpharmacological measures in patients with severe, refractory symptoms. Recommended drugs have also been extensively used where symptoms persist. These include:
- volume expanders (fludrocortisone, desmopressin, and intravenous saline)
- heart rate inhibitors (propranolol, ivabradine, and pyridostigmine)
- vasoconstrictors (midodrine, octreotide, methylphenidate, and droxidopa)
- sympatholytic drugs (clonidine and methyldopa)
Decisions regarding which treatment to initiate should be guided by specific symptoms and hemodynamic patterns, i.e., tachycardic vs. hypotensive phenotypes.
The tachycardic phenotype can be treated with beta-blockers, i.e., metoprolol, or ivabradine. Recently, intravenous metoprolol has been tested for use in the treatment of acute respiratory distress in acute COVID-19 and was found to improve oxygenation and reduce alveolar inflammation, shortening the duration of invasive mechanical ventilation overall [92]. Large-scale randomized evidence is eagerly awaited to [figure out if the drug is helpful or not].
In patients with the hypotensive phenotype, droxidopa, midodrine, or pyridostigmine may be considered. In hypovolemic patients, intravenous saline infusion and intravascular volume expansion would be highly desirable, while the use of fludrocortisone and desmopressin should be reserved to patients with severe refractory symptoms. Sympatholytic drugs, such as clonidine and methyldopa, can be proposed to patients with hyperadrenergic features, including hyperhidrosis and tachycardia.
Beyond these drugs, immunological therapy with intravenous immunoglobulins [IVIG] has been proposed for compassionate use in a patient with autoimmune features
Diagnosis protocol: Figure 2 in the paper shows a diagnosis algorithm to differentiate between PASC (long COVID) and other conditions that may resemble it. Some of the tests include:
- CRP (C-reactive protein) / PCT (procalcitonin) - markers of systemic inflammation and bacterial infection
- NT-proBNP - High levels can mean your heart isn't pumping as much blood as your body needs → heart failure
- TSH - thyroid stimulating hormone levels indicate a healthy/unhealthy thyroid
- Morning cortisol - cortisol level may show problems with the adrenal glands or pituitary gland
- Holter ECG - This portable device tracks the patient’s heart rhythm over 1 or 2 days.
- 24-hour ambulatory blood pressure monitoring - portable device that tracks blood pressure.
- Exercise testing
- Valsalva maneuver - a breathing method that may slow your heart when it's beating too fast.
- Auto-antibody testing if known or suspected autoimmune condition
- Serum (blood levels of) histamine and tryptase testing if MCAS suspected. MCAS symptoms include flushing, headache, GI symptoms.
Stellate ganglion block[edit]
The stellate ganglion is a collection of nerves that are part of the autonomic nervous system / sympathetic nervous system, which directs the body's rapid involuntary response to dangerous or stressful situations. "Block" refers to injecting a local anesthetic to block the activity of this set of nerves, allowing the regional autonomic nervous system to "reboot". It may help treat symptoms related to dysautonomia. The experimental treatment has been used prior to COVID by different doctors on various conditions.
A paper by Luke D Liu and Deborah L Duricka (https://doi.org/10.1016/j.jneuroim.2021.577784) presents a case series of two long COVID patients treated with a stellate ganglion block. Their outcomes were reported in figures 1 and 2 of the paper (click the thumbnails on the right to enlarge the figures). The paper notes that the mechanisms "for the durable central nervous system effects of the SGB in these conditions are unclear, delaying its broad acceptance as a valid treatment".
It is likely that the clinic has seen many patients and that the case reports only selectively highlight results from highly satisfied patients. As well, retrospectively-recalled outcomes and retrospective observational studies may have biases in their data.
INUSpheresis / Therapeutic apheresis[edit]
A 2023 paper by Achletiner, Steenblock et al. (DOI:10.1038/s41380-023-02084-1) argues that certain biomarkers (autoantibodies, lipids, inflammatory markers, blood analysis via dark field microscopy) are reduced following INUSpheresis.
A 2022 paper by Steenblock et al. (DOI:10.1055/a-1945-9694) presents data from the same INUSpheresis clinic in Cham Germany.
Included were 1111 patients (2009–2022) with ME/CFS (148 following COVID-19, 963 other infections (e. g., Lyme disease, toxoplasmosis, EBV, or chlamydia), environmental factors (e. g., organic solvents) or unknown cause). However, no placebo controls were included. Following this protocol, 56% of the patients reported to be without symptoms or substantially improved following 2nd INUSpheresis (TKM58), 64% were without symptoms or substantially better following 3rd INUSpheresis (TKM58 or INUS 30) and additional therapy (prednisolone or vitamin C), and 74% were without symptoms or significantly better 6 months after INUSpheresis with follow-up therapy. Eleven percent of the ME/CFS patients experienced a moderate improvement and 15% did not encounter an improvement.
Monoclonal antibodies[edit]
Scheppke and colleagues (casirivimab/imdevimab cocktail)[edit]
Scheppke and colleagues report that 3 Long COVID patients experienced "complete remissions within days of MCA treatment".
https://www.sciencedirect.com/science/article/abs/pii/S073567572300534X
Aerium Therapeutics[edit]
Persistent viral infection with viral reservoirs and detection of circulating spike protein after the initial acute illness is one potential pathogenic mechanism for Long COVID. This mechanism may be able to be targeted by SARS-CoV-2 monoclonal antibodies (mAbs). This trial will study the safety and efficacy of AER002 to treat individuals with Long COVID in an adult population. https://classic.clinicaltrials.gov/ct2/show/NCT05877508
Mary Bowden[edit]
She uses monoclonal antibodies in addition to the FLCCC protocols to treat long haul (long COVID and post vaccination syndrome). See the 25 minute mark of this video: https://odysee.com/@FrontlineCovid19CriticalCareAlliance:c/FLCCC-WEBINAR-120121_FINAL:f?r=9U5LyeCuf2jpinYRxYjvNdaM6nQqsThF&t=1502 Bowden is based in Houston TX. breathemd.org
Keith Berkowitz / intravenous vitamin C / LDN[edit]
In addition to other drugs/treatments like ivermectin, he uses intravenous vitamin C for the treatment of long COVID. See the 33 minute mark of this video - https://youtu.be/hEcQ2SdwEmI?t=1979
He uses low-dose naltrexone for MCAS (mast cell activation syndrome).
SSRIs[edit]
This study probably didn't study Long COVID as 12.5% developed post-Covid-syndrome (PCS).
https://www.nature.com/articles/s41598-023-45072-9
This study used an exploratory questionnaire and found that two-thirds of patients had a reasonably good to strong response on SSRIs, over a quarter of patients had moderate response, while 10% reported no response. Overall, patients experienced substantial improved well-being. Brainfog and sensory overload decreased most, followed by chronic fatigue and dysautonomia. Outcomes were measured with three different measures that correlated strongly with each other.
Nicotine patches (Marco Leitzke)[edit]
Treating several individuals suffering from post-COVID-19 syndrome with a nicotine patch application, we witnessed improvements ranging from immediate and substantial to complete remission in a matter of days.
https://bioelecmed.biomedcentral.com/articles/10.1186/s42234-023-00104-7
BMJ treatment guidelines[edit]
Many mainstream doctors will follow the treatment guidelines outlined in the British Medical Journal: https://www.bmj.com/content/370/bmj.m3026
Key treatments: None (!!!). The guidelines argue that doctors shouldn’t try because “There are not yet definitive, evidence based recommendations for the management of post-acute covid-19.”
If a patient has blood clotting issues, the guidelines highlight “prophylactic anticoagulation”- giving anti-clotting drugs to prevent future blood clots.
Significance: The medical establishment believes that doctors should avoid trying to treat the patient if they encounter weird health problems that they don’t understand.
🇺🇸 CDC recommendations[edit]
According to the CDC's webpage on the management of post-COVID conditions (current version, archived version), the CDC believes that patients may benefit from certain types of unproven treatments but not others.
- A conservative exercise-based rehabilitation plan may be indicated for patients with post-exertional malaise (symptoms that worsen following too much physical or mental exertion).
- Symptom management approaches for ME/CFS, fibromyalgia, post-treatment Lyme disease syndrome, dysautonomia, and MCAS may be useful in patients who share some of the symptoms.
Otherwise, doctors should discourage their patients from using other treatments that have been offered because they "lack evidence of efficacy or effectiveness, and could be harmful to patients". Healthcare professionals should inquire about any unprescribed medications, herbal remedies, supplements, or other treatments. Presumably, this is to discourage the patients from trying the 'wrong' treatments.
Doctors should also avoid extensive testing of long COVID patients due to "the increased risk for incidental findings, anxiety about abnormal results that do not have clinical significance, imaging-related radiation exposure, and cost" (archived version). However, testing should not be delayed when there are signs and symptoms of urgent and potentially life-threatening conditions (e.g., a blood clot in an artery in the lung, heart attack, pericarditis with effusion, stroke, kidney failure).
Experimental treatments/drugs in clinical trials[edit]
Experimental drugs tend to be very difficult to get, so they may not be very relevant to patients. They generally do not have an established safety track record.
Lists of clinical trials[edit]
https://crunchme.notion.site/4d00e39f9f874a4c85cb9046149aeba7?v=2c3284eb773a403e817db9598480a768
Baractinib[edit]
Baractinib is an immunomodulatory medication used for the treatment of rheumatoid arthritis, alopecia areata, and COVID-19. Wes Ely is spearheading a pilot study for REVERSE-LC, "a phase 3 trial of baricitinib versus placebo in adults with neurocognitive impairment (a form of Alzheimer's Disease and Related Dementias or ADRD) or cardiopulmonary symptoms due to Long COVID".
https://classic.clinicaltrials.gov/ct2/show/NCT05858515
Paxlovid - Stanford, Yale, NIH RECOVER[edit]
Stanford studied Paxlovid (Nirmatrelvir/ritonavir) in a clinical trial - see NCT05576662. A MedPage Today article states that trial enrollment was stopped early.
Two sources familiar with the STOP-PASC study told MedPage Today that trial enrollment had been halted. One was told by a study coordinator that a preliminary review found "inconclusive evidence" for the primary outcome of the study. Another said their first appointment was canceled just a few days before it was supposed to take place, and they were later told that all future enrollment had been halted.
Yale is currently recruiting for a similar study on Paxlovid in Long COVID patients (NCT05668091).
The NIH announced its RECOVER-VITAL study which will "test a longer dose regimen of the antiviral PAXLOVID (nirmatrelvir and ritonavir) than is used for treating acute COVID to see if it improves the symptoms of patients with long COVID".
Remdesivir[edit]
A UK team at the University of Derby will study whether remdesivir would be useful in those with Long COVID.
Remdesivir and its metabolite (GS-441524) have been tried by ME/CFS patients. https://forum.sickandabandoned.com/t/different-causes-of-chronic-illness-me-cfs-and-john-chias-success-in-treating-his-son/238
Valacyclovir and Celecoxib[edit]
Virios Therapeutics announced results from an open-label (not blinded) proof of concept study in a July 17 2023 press release. Health Rising has a summary of the approach and the theory behind it.
Leronlimab[edit]
Leronlimab is a CCR5 antagonist so its theoretical mechanism of action is the same as maraviroc (a drug used by the Bruce Patterson / covidlonghaulers.com group). Cytodyn's study of leronlimab on long COVID did not reach statistical significance (source: Cytodyn press release). Cytodyn claims that the trial was not designed to reach statistical significance and notes that the sample size was 56 patients. A larger trial would provide more evidence as to whether or not this drug (and perhaps CCR5 antagonists in general) is helpful.
Bruce Patterson was involved in a group of investors that was trying to take control of Cytodyn, the company which owns the right to this drug.
Gaylis and colleagues have published a pre-print on leronlimab for long COVID (https://doi.org/10.1093/cid/ciac226). Spplementary Figure 1 contains data from the RCT.
LAU-7b[edit]
ESSOR is a double-blind, placebo-controlled study of the orally-administered antiviral and inflammation-controlling LAU-7b for the treatment of adults with Long COVID and moderate to severe symptoms.
https://clinicaltrials.gov/study/NCT05999435
RSLV-132[edit]
RSLV-132 is a protein designed to 'digest' RNA that is circulating in the blood, hopefully leading to a reduction in inflammation. Clinical trials started years ago with the intent of developing a drug to use against autoimmune conditions such as Sjogren's Syndrome (e.g. NCT03247686). The company does not seem to be pursuing a phase III trial for Sjogren's Syndrome. See https://resolvetherapeutics.com/product-candidates/rslv-132/
NCT03247686[edit]
The present study will examine the role of circulating RNA complexed with autoantibodies and immune complexes and its role in activation of inflammatory pathways in patients with primary Sjogren's syndrome. The study will be conducted in a subset of Sjogren's patients who have elevated levels of autoantibodies and a pattern of elevated interferon-stimulated gene expression in blood cells. A number of biochemical and clinical parameters will be analyzed to determine the potential therapeutic utility of nuclease therapy in Sjogren's syndrome.
https://clinicaltrials.gov/ct2/show/NCT03247686
NCT04944121[edit]
Phase 2 Study of RSLV-132 in Subjects With Long COVID
https://clinicaltrials.gov/ct2/show/NCT04944121
AXA1125[edit]
AXA1125 is a drug originally developed to see if it is viable for the treatment of NASH, or 'fatty liver disease'. The drug is being trialed to see if resolving mitochondrial dysfunction will heal the fatigue associated with long COVID. See https://axcellatx.com/pipeline/axa1125/
On August 2 2022, Axcella announced that its phase II clinical trial did not find statistically significant results for its primary endpoint (phosphocreatine recovery time [PCrτ] following moderate exercise as assessed by 31P-magnetic resonance spectroscopy). See the press release titled Axcella Announces Highly Promising Results from Phase 2a Placebo Controlled Clinical Trial for Long COVID as well as plain-language summaries by pharma media outlets such as Endpoints News.
BC 007[edit]
BC 007 is designed to eliminate auto-antibodies against G protein-coupled receptors. Such auto-antibodies are found in patients with advanced heart failure, dilated cardiomyopathy, myocarditis, long COVID, dementia, ME/CFS, POTS, glaucoma, and other conditions.
BC 007 has yet to be proven for any health condition, although Berlin Cures has published case studies on individual patients.
- Case Report: Neutralization of Autoantibodies Targeting G-Protein-Coupled Receptors Improves Capillary Impairment and Fatigue Symptoms After COVID-19 Infection https://www.frontiersin.org/articles/10.3389/fmed.2021.754667/full
- Functional autoantibodies in patients with different forms of dementia https://doi.org/10.1371/journal.pone.0203253
- The aptamer BC 007 for treatment of dilated cardiomyopathy: evaluation in Doberman Pinschers of efficacy and outcomes https://doi.org/10.1002/ehf2.12628
- Functional autoantibodies in patients with different forms of dementia https://doi.org/10.1371/journal.pone.0192778
Results in humans[edit]
A compilation of anecdotes is available in the Sick And Abandoned forum: https://forum.sickandabandoned.com/t/bc007-anecdotes-so-far-not-the-silver-bullet-that-were-looking-for/337
The anecdotes from the clinical trials and another BC007 source suggests that the treatment does not work for most people, though these anecdotes are unverified.
Oxaloacetate[edit]
Kaufman and Cash ran a trial on oxaloacetate for ME/CFS and Long COVID patients. However, this trial has been criticized for its study design, unconventional data analysis, and conflicts of interest.
- https://twitter.com/CyruxiME/status/1561061393876619265 - Reanalysis of the oxaloacetate trial for #LongCovid and #MECFS
- https://doi.org/10.1186/s12967-022-03488-3 - Oxaloacetate Treatment For Mental And Physical Fatigue In Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Long-COVID fatigue patients: a non-randomized controlled clinical trial
Vagus Nerve Stimulation[edit]
Badran and colleagues published the results of their RCT on Long COVID patients (DOI:10.1186/s42234-022-00094-y). As the study consisted of only 13 patients, it was underpowered to detect any statistically significant benefit.
Dolphin VNS and marketing claims[edit]
Azabou and colleagues (DOI:10.3389/fmed.2021.625836) have suggested that vagus nerve stimulation may be useful in the treatment of acute COVID. The paper does not mention long haul, PASC, or Post-COVID syndrome. A company called Dolphin Neurostim has cited the paper to support the use of VNS in patients who have both acute COVID and worsened long-haul symptoms.
Health Canada has approved the device for patients "with known or suspected COVID-19" and "are experiencing exacerbation of longhaul symptoms and/or asthma-related dyspnea and reduced airflow [...]".
Ampligen / rintatolimod[edit]
Ampligen is a TLR3 agonist that activates the part of the body's innate immune system that fights viruses. As such, it has immunomodulatory properties.
Phase 2 study: https://classic.clinicaltrials.gov/ct2/show/NCT05592418?term=AMP-518&draw=2&rank=1
MEPedia has more information on the drug as it has been in development for ME/CFS since the 1990s. https://me-pedia.org/wiki/Ampligen
Tonix Pharmaceuticals TNX-102 SL[edit]
TNX-102 SL is a patented sublingual tablet formulation of cyclobenzaprine hydrochloride which provides rapid transmucosal absorption and reduced production of a long half-life active metabolite, norcyclobenzaprine, due to bypass of first-pass hepatic metabolism. As a multifunctional agent with potent binding and antagonist activities at the 5-HT2A-serotonergic, α1-adrenergic, H1-histaminergic, and M1-muscarinic receptors, TNX-102 SL is in development as a daily bedtime treatment for fibromyalgia, Long COVID (formally known as post-acute sequelae of COVID-19 [PASC]), alcohol use disorder and agitation in Alzheimer’s disease.
TNX-102 SL did not meet its primary endpoint in its phase 2 clinical trial. See the Sept 05, 23023 press release.
Efgartigimod (Vyvgart)[edit]
This drug by Argenx is approved for Myasthenia Gravis, a chronic autoimmune disorder. It targets the neonatal Fc receptor (FcRn) and is being trialed for other autoimmune conditions such as lupus and Sjogren’s Syndrome. Its developer is also trialing it for POTS caused by COVID.
Health Rising writeup: https://www.healthrising.org/blog/2023/01/13/long-covid-clinical-trials-big-drugs-big-studies-and-much-more/
Ibudilast and Pentoxifylline[edit]
Ibudilast is an anti-inflammatory that vasodilates the blood vessels, has neuroprotective effects, inhibits platelet aggregation, and suppresses microglial cell activation.
Pentoxifylline is a xanthine derivative that’s used to treat muscle pain in people with blood vessel problems and peripheral neuropathy.
Both are being trialed in Canada. Health Rising writeup: https://www.healthrising.org/blog/2023/01/13/long-covid-clinical-trials-big-drugs-big-studies-and-much-more/
Temelimab (Imjudo)[edit]
Tremelimumab is designed to attach to and block CTLA-4, a protein that controls the activity of T cells, which are part of the immune system (the body’s natural defenses).
The GNC-501 study, entitled “Temelimab as a Disease Modifying Therapy in Patients with Neurological, Neuropsychological, and Psychiatric Symptoms in Post-COVID-19 or Post-Acute Sequelae of COVID-19 (PASC) Syndrome”, will enroll 200 patients from Swiss and EU study centres suffering from severe neuropsychiatric syndromes following COVID infection. The biomarker-based study will enrol only patients who are also tested positive for the pathogenic protein W-ENV, with the objective to reduce their invalidating conditions.
- https://clinicaltrials.gov/ct2/show/NCT05497089?term=temelimab&draw=2&rank=1
- https://geneuro.com/en/Long-COVID/herv-in-long-COVID/new-clinical-trial-in-long-COVID
Anti-KIT monoclonal antibody CDX-0159[edit]
CellDex Therapeutics believes that their monoclonal can target mast cells and suppress them. https://pubmed.ncbi.nlm.nih.gov/35184297/
Periodic Fasting and Time-restricted eating for Long Covid[edit]
Professor Jeffrey Novack is the primary investigator on an active clinical trial, "Diet and Fasting for Long Covid". Thomas Bunker, an Immunologist and Cell Biologist who is a Long-hauler himself, is the co-investigator. This trial uses patient reported symptoms and severities to calculate a Long Covid Score. How the severity of participants Long Covid changes over the 4 weeks of a no added sugar diet and 10 hr time-restricted eating (TRE) is being comparted to how symptoms change over 4 weeks of a no added sugar diet and a one or two day water fast each week.
There are no conficts of interest as these are no cost "self-treatments" that anyone can do, anywhere in the world. The main purpose of the trial is to establish safety and efficacy of these potential self-treatments.
The underlying hypothesis of the study is that Long haulers have a persistent low-level SARS-CoV-2 infection that causes all symptoms. TRE and a no added sugar diet improve cellular metabolic health. While 36 to 60 hour water fasts strongly induce the intenal cellular housecleaning pathway of autophagy. Autophagy makes viral infection more visible to the immune system and may flare up symptoms for a day or so.
To learn more, download the official study flyer pdf at https://recoverfromlongcovid.com/diet-and-fasting-for-long-covid/
Lactoferrin[edit]
Studied in a randomized controlled trial.
Lactoferrin provided no benefit in terms of fatigue, other PROMs or physical functioning.
https://openres.ersjournals.com/content/early/2024/03/21/23120541.00031-2024
Comprehensive list of Long COVID trials[edit]
https://docs.google.com/spreadsheets/d/1_JNYk5fEldrfcrgnZVuMi3_d5dfNdHYo0mXKmEQgwrQ/edit?usp=sharing
Maintained by Siobhan Bligh.
Other conditions that might resemble long haul[edit]
Patients should also consider the possibility that they may have a treatable condition that isn’t long haul. Unfortunately, many of these conditions may be difficult to diagnose.
Breast implant injury (BII)[edit]
As the name suggests, this only affects people with breast implants.
BII can affect anybody with an implant regardless of whether the implant is intact. It seems to affect all generations of breast implants. The treatment is straightforward: breast implant explantation (removal). There is controversy as to whether or not all of the scar tissue should be removed too.
While there is no test for BII, a MRI scan can reveal ruptured implants. A ruptured implant could drive the decision to have implants removed.
See the BII resources page for more information.
Iatrogenic botulism from Botox, Dysport, etc.[edit]
This condition can only affect people who have botulinum toxin in their body, e.g. due to Botox injections. In some people, the toxin can spread to other areas of the body and affect nerve function outside of targeted areas. This can result in symptoms such as:
- Difficult swallowing (dysphagia)
- Drooping of the upper eyelid (ptosis)
- Diplopia (double vision)
- Systemic weakness
- Muscle paralysis
The Facebook group 'Botox Dysport (Side Effects) Support' has resources on this condition, with references to published scientific papers.
Medical devices, joint replacements, IUDs, and other foreign objects in the body[edit]
Medical devices and artificial joints can lead to an infection from bacteria or fungi such as Candida Albicans, in turn leading to chronic health problems.
Essure is a permanent birth control device that is no longer being sold. It was featured in the 2018 Netflix documentary The Bleeding Edge and was pulled off the US market shortly after the documentary premiered. Facebook support groups exist for Essure.
Chronic Lyme (and Lyme+)[edit]
There are various ways in which you can pick up a persistent infection of the various bacteria associated with Lyme disease. Lyme+ refers to all pathogens that can cause serious health problems and share similarities with chronic Lyme: difficult to diagnose and difficult to treat.
There is controversy as to whether or not chronic Lyme exists. For a good introduction to the topic, see DrBeen’s conversation with Steven Philips on Youtube: https://youtu.be/FqVlOfzZJH0
Lyme disease can be diagnosed by:
- Taking multiple photos of the tick bite. However, most people do not have a tick bite
- Blood tests. However, the blood tests aren’t reliable and have a very high rate of false negatives.
- Diagnose based on symptoms. (This diagnosis method isn’t reliable.) Typical symptoms include fever, headache, fatigue, and a characteristic “bullseye” skin rash called erythema migrans. See https://www.lymedisease.org/lyme-disease-symptom-checklist/ for a diagnosis checklist.
- Getting treated with a course of antibiotics too see if the patient develops a Jarisch-Herxheimer (“Herx”) reaction. The reaction is unpleasant and may lead to hospitalization.
Patient advocacy organizations like LymeDisease.org will have lots of information on chronic Lyme, its treatments, and information on how to find a ‘Lyme literate medical doctor’ specializing in this condition. Treatment may require several years and does not always succeed.
Autoimmune conditions affecting the thyroid or brain[edit]
Hashimoto’s disease is an autoimmune condition where the immune system attacks the thyroid gland. Its symptoms include:
- Fatigue and sluggishness
- Increased sensitivity to cold
- Depression
Autoimmune encephalitis is a very rare condition where the immune system attacks the brain. It causes moderate deficits of memory and cognition, often followed by suppressed levels of consciousness or coma. It can be misdiagnosed as schizophrenia.
If you have symptoms of either autoimmune condition, further investigation may be helpful. Thyroid hormone levels in the blood can be tested. The NMDA receptor antibody test can detect autoimmune encephalitis. However, many doctors may be unfamiliar with that test. For more information, you can read the book Brain on Fire or its Netflix adaptation.
One way to treat and test for autoimmune conditions is to try either of the following diets: Water fasting. Drink water and don’t eat for a few days. A meat-only elimination diet (“carnivore diet”). You can find plenty of information about these diets online. They may have dangerous interactions with medications (e.g. because the diet can reverse underlying conditions and the drugs suddenly become dangerous when the underlying condition such as diabetes goes away). They likely have interactions with psych meds.
The Paleomedicina group of doctors in Hungary treats autoimmune conditions through diet. They provide online consultations and should have experience with the safe discontinuation of medications.
HPV vaccine injury (Gardasil, Cervarix) and vaccines with aluminium-based adjuvants (e.g. anthrax)[edit]
These vaccines have safety controversies somewhat similar to the COVID vaccines. Japan's usage of HPV vaccines has dropped to almost zero.
[edit]
A Chiari malformation refers to a part of the brain (cerebellum) that dangles down into the spinal column instead of holding its normal rounded shape. This unusual structure can be detected via a scan of the brain (e.g. MRI, CT, etc.). Because some people have a Chiari malformation without symptoms, diagnosis is based on both symptoms and an abnormal brain scan.
Chiari support groups and forums will have additional information on this condition.
- https://www.chiarisupport.org/
- https://www.chiariassociation.org/ has a list of doctors with experience in treating Chiari
The prevalence of Chiari, CCI, and hypermobile EDS tends to overlap. People with unusually flexible joints (without stretching or contortionist training) may be more likely to fit the diagnostic criteria for hypermobile EDS and Chiari.
Tethered cord syndrome[edit]
Tethered cord syndrome is a rare condition in which the spinal cord is 'tethered' (attached) to the surrounding tissues of the spine. People with this condition may find it easier to walk using their toes and without using their heel ("toe walking").
ME/CFS[edit]
The tests for myalgic encephalitis / chronic fatigue syndrome aren’t great and neither are the treatments. Patients may want to rule out everything else first.
An interview with Dr. John Chia - https://youtu.be/LSucgoEvoUQ - outlines some tests and treatments for enterovirus infections. (*Note: Chia mentions remdesivir, a deadly drug that killed 1-3 patients in the French DisCoVeRy trial. Be careful with that drug because there must be a reason why its manufacturer abandoned its clinical trials for using remdesivir in early COVID treatment.)
ME-pedia has an overview of symptom management of ME/CFS - https://me-pedia.org/wiki/Primer_for_patients#Drugs_and_treatments